
Features
Structures & Equipment
Water and irrigation
Raising low medium-pH
March 20, 2012 By Dr. Olu Kamson
Soilless media horticulture relies extensively on naturally occurring materials
Soilless media horticulture relies extensively on naturally occurring materials such as peat, bark, compost, and to a lesser extent, on inorganic materials such as perlite, vermiculite, rockwool, etc.
 |
|
| A treated plant, at left, with an untreated plant. PHOTOS COURTESY WILLOWBROOK NURSERIES Advertisement
|
Most soilless media components are acidic in nature and growers are more frequently concerned with raising medium-pH. Several options are available for raising low medium-pH. These include switching to a nitrate-based fertilizer, stopping acidification of irrigation water, and applying flowable lime, potassium bicarbonate, hydrated lime, among others.
Extensive evaluation of flowable lime (Limestone F) and potassium bicarbonate (KHC03) for quickly raising medium-pH on bedding plant crops after planting has been reported by Argo and Fisher1. Medium-pH increases of up to 1.0 unit of pH were reported using flowable limestone (10 ml/L) or 2.4 g/L of potassium bicarbonate. These authors further reported that potassium bicarbonate raised medium-pH by 1.5 units within 24 hours while the full effect of flowable lime was not achieved until a week later. They also mentioned that response of chemicals depended on media type.
 |
|
| Hibiscus plants used in the study. |
In this paper, the procedure and results of a method, involving Total Immersion Medium-pH Adjustment (or TIMPA), which uses potassium bicarbonate to permanently raise medium-pH within one hour, is presented. The method is fast, highly reproducible and can be designed for obtaining a desired medium-pH. The method has been successfully applied for adjusting pH of our in-house formulated media (standard Willowbrook mix) as well as commercially available growing media. The procedure can be applied to individual container pots (or trays) before and/or after planting.
MATERIALS AND METHODS
■ Media composition and characteristics
Low pH media consisting of bark, peat and perlite were initially used (without plantings) for medium-pH adjustments. Subsequently, container pots with plantings (Hibiscus diana and Euonymous emerald gaiety) growing under low medium-pHs were selected and taken through the procedure.
Commercially available media: OASIS Fertiss 105-pack trays (with and without Clematis allanah cuttings) were also taken through the medium-pH adjustment procedure described below.
pH AND EC MEASUREMENTS
■ Pour-through method of media nutrient extraction was carried out on all media investigated and extracts obtained were used directly for pH and EC determinations. All pH and EC measurements were carried out using an Oakton pH/CON 300 Deluxe pH/conductivity meter with pH resolution of ± 0.01 pH. The meter was freshly calibrated each time it was switched on. All electrical conductivity (EC) measurements in this report are in mS units.
POTASSIUM BICARBONATE SOLUTION
■ Prepare sufficient 4.0 g/L potassium bicarbonate solution for total immersion of pots (or trays) containing media whose pH is to be adjusted. This is done by dissolving completely the required amount of dry salt in the volume of water necessary for total immersion of the pots (or trays) in the bucket to be used.
TOTAL IMMERSION OF TEST MEDIA
■ Slowly (and gently) lower the pot (or tray) containing media (with or without planting) into bucket of potassium bicarbonate solution until media’s topmost layer is just below the solution line. Wait for the bicarbonate solution to come trickling up spontaneously to the media surface as it displaces air filled pore spaces in the media. During the wait period, saturation of media’s active sites takes place by countercurrent motion of potassium and bicarbonate ions.
Once bicarbonate dip solution appears as a layer on media surface, allow ongoing ion exchanges between media active site ions and potassium and bicarbonate ions to take place undisturbed for at least one hour. Remove media-containing pot (or tray) from dip solution and allow excess bicarbonate solution and exchanged ions to drain freely under gravity through drain holes in the pot (or tray).
After most of the excess bicarbonate solution (and exchanged ions) have drained from the pot (or tray), leach un-adsorbed potassium and bicarbonate ions from the medium using irrigation water. Leach using an amount of water equivalent to the volume of the pot (or tray).
Determine medium-pH using a rapid procedure (pour-through) to confirm the new medium-pH attained as well as to ensure that the EC of pH-adjusted media is within acceptable limits. Otherwise, leach again using the same amount of irrigation water to bring the EC within limits.
TARGETING A DESIRED MEDIUM-PH
■ The pot (or tray) containing media is immersed and re-immersed in either spent (or freshly prepared) bicarbonate dip solution to bring about successive ion exchange at substrates’ active sites until desired medium-pH is attained. This involves carrying out consecutive pH tests after each total media immersion to confirm medium-pH attained compared to desired medium-pH.
Once the target pH has been attained, reduce medium EC levels by leaching once or twice using appropriate amounts of irrigation water.
|
RESULTS AND DISCUSSION
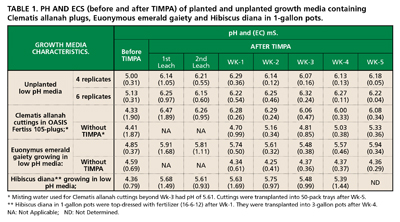
■ The TIMPA method described above was applied to plants growing in low pH media (one-gallon pots) and fresh Clematis cuttings in OASIS Fertiss 105-pack trays. Using the method, up to 2.0 units of medium-pH gain was achieved for growing media (with or without plantings) within one hour (Table 1). The table shows that during the five-week observation period, subsisting medium-pHs were within optimum for plants (and cuttings) grown in TIMPA-treated media. This was not the case for plants grown in media without TIMPA.
The medium-pH gain is permanent, which implies that some acidic active sites on the media have been neutralized. Although the exact mechanism of ion exchange at media’s active sites was not investigated, it is clearly facilitated by countercurrent motion of bicarbonate and potassium ions within the media. This is evidenced by the slight lowering of the pH of the bicarbonate dip solution after the reaction has taken place. In fact, the bicarbonate dip solution can be further reused for adjusting medium-pH of other pots (or trays) with substantial pH gain as long as dip solution’s pH is not less than 7.5units. If dip solution’s pH is below 7.5, then more solid KHC03 can be added to bring it above 8.0 and it can then be used again.
The advantages of this method of medium-pH adjustment are many. Firstly, the medium-pH is adjusted within one hour. Secondly, the medium-pH gain is permanent because it is based on an ion-exchange process. Excess potassium and bicarbonate ions are leached out readily (>50 per cent drop in EC) immediately after the first wash of growing medium. The method can be applied to the media before and/or after planting. If already planted pots (or trays) are used, there is no damage to lower foliage if the pot (or tray) is gently lowered into the dip solution.
If the method is applied before planting, then the plant plug is initiated in an optimum medium-pH environment for it to grow and develop. The unique attributes of this method can be taken advantage of by large-scale commercial growing media manufacturers.
The total immersion method precludes bicarbonate (or potassium) ions channelling straight through the media, which frequently occurs when basic chemicals are applied from the top of the media2. Channelling of treatment solution accounts for the erroneous and inconsistent medium-pH gains often obtained when bicarbonate solution is applied from the top of the medium. Conversely, the total immersion method produces permanent, consistently reliable and reproducible medium-pH gain when done properly. The amount of medium-pH gain achievable is dependent on media type, their particle size and the number of active exchangeable sites on the materials used for media formulation. The method can be applied for adjusting the pH of individual growing media components before mixing and potting them.
In addition, the method can be used for lowering growing medium-pH in cases where medium-pH concerns are on the alkaline side of the pH spectrum.
ACKNOWLEDGEMENT
John Langendoen, president and founder, Willowbrook Nurseries Inc., is hereby acknowledged for his commitment to, and very active support of, research at his farm.
REFERENCES
Understanding pH Management for Container-Grown Crops, William R. Argo and Paul R. Fisher, a Meister Publication.
What is your substrate trying to tell you, Part III, Robert R. Tripepi, Plant Science Division, University of Idaho, Moscow, ID 83844-2339, http://www.extension.uidaho.edu/nursery/Landscape%20problems/Substrate/Measuring%20pH%20and%20CEC.PDF
| Kamson a LEED pioneer Dr. Olu Kamson is a University of Birmingham, U.K.- trained analytical chemist with considerable expertise in ecological sustainability. He has been involved in a number of multidisciplinary, interdisciplinary and cross-functional research studies. He is highly skilled in the use of a very wide variety of analytical instrumentation. His research interests include method development for continuous flow systems, phyto-remediation of degraded ecosystems, and biosolids application in ornamental horticulture. He is currently Crop Research Scientist at Willowbrook Nurseries Inc., Fenwick, Ontario, and adjunct professor of environmental management at the University of Fredericton. Kamson is a pioneer fellow of LEED International, the largest professional network in the world devoted exclusively to sustainable development. |
 |
Print this page