
Features
Fertilizer
Inputs
Grower’s Toolbox: Viewing the differences between alkalinity and pH
June 28, 2008 By Albert Grimm
When you look at irrigation water
samples, what is the difference between alkalinity and pH, and why is
that difference so significant? I’ll try a very unscientific approach
to explain this, and I hope it will nonetheless clarify at least the
principles. Just don’t show this to your kid’s high school chemistry
teacher … please!
 |

|
 |
 |
| MONITORING Regular monitoring of pH and alkalinity helps growers provide optimal growing conditions. |
WATER CHECKS The difference between irrigation water alkalinity and pH is significant. |
pH LEVELS Soil pH levels determine which chemical reactions can take place easily and which are inhibited. |
IN BALANCE Roots manage the chemical reactions in the soil to create a balance for the nutrients that are taken up. |
When you look at irrigation water samples, what is the difference between alkalinity and pH, and why is that difference so significant? I’ll try a very unscientific approach to explain this, and I hope it will nonetheless clarify at least the principles. Just don’t show this to your kid’s high school chemistry teacher … please!
What is pH: One standard explanation is that the pH value expresses the degree of acidity of the water in our substrates. This does, of course, not help much, because apart from the fact that acids taste sour, we still don’t know what the significance of the acidity is – and we are not planning to eat the soil from our poinsettias, either.
CALCULATING PONDUS HYDROGENII For those that are interested (and this should stand up even to a very critical chemistry teacher), please note the following.
 Firstly, pH stands for Pondus Hydrogenii, and expresses the concentration of H3O+ ions in an aqueous solution. For those who are fit in mathematics: the pH is the negative, decadic logarithm of the H3O+ ion concentration. Firstly, pH stands for Pondus Hydrogenii, and expresses the concentration of H3O+ ions in an aqueous solution. For those who are fit in mathematics: the pH is the negative, decadic logarithm of the H3O+ ion concentration.
H3O+ ions form from water molecules when an acid “parks” protons in water until they are used up by something else. The more acid in water, the more H3O+ ions can be counted. The calculation works like this: In pure water, at room temperature, we find approximately one H3O+ ion for every 10 million water molecules. Mathematically, we can express the relationship as shown in accompanying chart. Because of this number game, the pH of neutral water is 7.0 at room temperature. Now, if we pour a lot of acid into the water, we add available protons, and those can react with water molecules to form H3O+ ions. As an example, let’s say we add enough acid so that we have as many as one H3O+ ion for every 100 water molecules. The number 100 is the same as 102. The concentration of H3O+ ions will therefore be 10-2. The negative value of the logarithm to the base 10 of 10-2 is 2. The pH of a solution that has one H3O+ ion for every 100 water molecules is two, and that is very acidic.
|
In very simple terms (chemistry teachers, please look away), I’ll offer the following description.
Most of the stuff in our universe is made from atoms. It is quite acceptable to picture atoms as small spheres, where tiny cannonballs of energy, called electrons fly in circles around a nucleus, which is made up of other tiny cannonballs called protons. An atom needs the same number of electrons and protons to be in balance. If an atom does not contain the same number of electrons and protons, it becomes electrically charged, and all kinds of interesting things start to happen, but that’s a different story we need not go into here. The problem with these electrons flying in circles around the atom’s nucleus is the fact that they have to come in pairs, otherwise the whole atom is not stable (chemistry teachers, now close your eyes, please). You can visualize this by picturing a spinning bicycle wheel with some weights attached to the rim. If you attach only one weight, the wheel wobbles pretty badly. However, if you place two weights of equal size on exactly opposite sides of the rim, the wheel will turn just fine. I know this is far fetched, but something like this happens in an atom.
The dilemma is that the atom cannot just add or remove electrons to balance all the spinning cannonball pairs, because then it becomes electrically charged and turns into an ion. Ions are very stable units without any “wobbling” among the electrons, but they are electrically charged and behave totally different than the original atom.
So, atoms do the next best thing – they look for another atom that has the same “wobble-problem,” and they share and pair up their electrons. Instead of individual electrons orbiting around each nucleus, the new arrangement is for both atoms to share an orbit and have the electrons circle around both nuclei, which sort of ties the two atoms together. The whole thing is now stable, without “wobbling”, and without electrical charge.
When that happens, and two atoms form a molecule, we call this a chemical reaction. Essentially, the entire science of chemistry does little more but investigate all the different ways of electron sharing and exchanging that happens between atoms for the purpose of wobble avoidance. (By now, any chemistry teacher still reading this probably went for some blood pressure pills, or thinks I am nuts, or both.)
And here is why I spend so much time explaining these atomic cannonballs: an acid is defined as a substance that either makes protons available to other molecules, or as something able to accept (use up) extra electrons from another molecule during chemical reactions.
This is pretty important stuff, if you consider that all chemical reactions involve the exchange of electrons and balancing them with available protons. The more acid molecules we add to water, the easier it is for other atoms and molecules to get rid of electrons or to find protons. Some chemical reactions cannot take place, unless there is a place available for surplus atomic cannonballs. Likewise, if chemicals need to find some extra electrons to get a reaction going, this becomes tougher, the more acid has been added to the water.
| WHAT DOES THIS MEAN TO THE PLANT? As growers, we have to help the plant by adapting its environment to be close to what it finds in its natural habitat. For the root environment, we can do that by regulating the alkalinity of the substrate and that of the irrigation water. The measurement of pH is a tool that helps us understand how easy or how hard it is for the root to do its job at any given time, and it tells us how effectively we are controlling the alkalinity. Measuring the alkalinity of the irrigation water tells us how much corrective action we have to take before the pH of the water changes, and the roots can work in a comfortable environment. Alkalinity and pH are somewhat related, but I hope that this little essay clarifies that it is very important to understand the differences. And I hope that all chemistry teachers who read this little piece will forgive me for the various little scientific sacrileges.
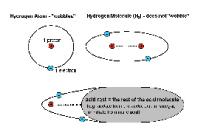 SOLUTIONS TO THE “WOBBLE-PROBLEM” SOLUTIONS TO THE “WOBBLE-PROBLEM”An individual hydrogen atom would be extremely reactive and unstable, because it has a lonely electron in its orbit. This graphic tries to illustrate nature’s solution to this problem. 1.) Two hydrogen atoms can bond together and share their electrons. Hydrogen gas in nature consists of such H2 molecules (at least under conditions in which humans can survive). 2.) The hydrogen atom can also bond the same way with another molecule if it has an individual electron available that can be shared to form an electron pair. In an acid molecule, however, the acid rest attracts the shared |
The pH value expresses nothing more than how many extra protons are available, or how many electrons can be accepted in a given water-based environment. This means that the pH determines which types of chemical reactions can take place easily, and which types of reactions are inhibited.
The roots of all plants are actively involved in managing chemical reactions in the surrounding soil, in order to create a balance for the nutrients that were taken up. If you take that into consideration, you may get some understandingas to why the pH of the soil solution has such a huge impact on how easy or how hard it is for plants to manage nutrient uptake.
With all this electron and proton exchanging going on, we may get atoms where the number of electrons does not match the number of protons. Such unbalanced atoms are called ions. Ions are stable but carry an electrical charge. Negatively charged ions with more electrons than protons are called anions, and positively charged ions with more protons than electrons are called cations. It is important to understand that you won’t find “electricity” in a glass of water that contains these electrically charged atoms,because there will always be the same number of positive and negative charges in a solution of ions.
One very special cation is that of hydrogen. A hydrogen atom has only one proton and one electron. So if you take the one electron away, you are left with just the proton. A proton by itself is even worse than a wobbly atom, so the proton from the hydrogen ion (H+) attaches itself to a water molecule (H2O) and forms the hydronium ion (H3O+). For this text, you don’t need to understand this, but when you read literature about pH and acidity, you will most certainly read about H3O+) ions, and about protons, or both. A hydrogen ion, and a proton are kind-of the same thing, but not quite.
WHAT IS ALKALINITY
Now, in contrast, what is the significance of the value for alkalinity that you find in your water analysis? Alkalinity expresses the concentration of carbonates and bicarbonates in water. Bicarbonates are ions that are able to react with available protons from the water, and neutralize them. If we add bicarbonates to pure water, we can slightly increase the pH of this water, so sometimes we confuse the pH reading of water with alkalinity.
The stuff that causes alkalinity in water is able to neutralize acids. The more carbonates or bicarbonates are dissolved in water, the more acid it takes to change the pH of the water. Alkalinity is the capacity of water to neutralize acids before the pH changes significantly.
For the grower, the significance of this story is a very practical application. When you want to change the pH of water by adding acid, the more alkalinity that is in the water, the more acid you will need to add before the pH of your water changes.
So, once more in simple words, the pH measures how much acid effect is present in the water, whereas the alkalinity determines how easy or how sluggish the water responds to any attempts of changing the pH.
HARDNESS
Hardness is yet another term that is somewhat related and is sometimes (wrongfully) thrown into the same pot as alkalinity and pH.
Certain dissolved ions in water, namely calcium, magnesium and iron, are able to form hard, insoluble deposits commonly known as scale. Scale is a hard, rock-like substance that we generally hope to avoid inside our boilers and water pipes (and office coffee makers, too). The term hardness describes the inherent capacity of dissolved ions to form such water-insoluble deposits. The harder the water, the more scale will form if the conditions are right. The softer the water, and the less hardness-causing ions in the water, the less chance there will be for your kettle to fail to boil.
Albert Grimm is the head grower at Jeffery’s Greenhouses in St. Catharines, Ontario.
Print this page