
Features
Growing Media
Inputs
Special Series: In the rootzone #6
Maintain a balanced nutrient composition and a controlled and stable pH and EC to achieve the best results
July 18, 2011 By Andrew Lee
Maintain a balanced nutrient composition and a controlled and stable pH and EC to achieve the best results
One of the benefits of using stonewool substrates is that the composition of the nutrient solution in the rootzone is under your total control. This is because stonewool is inert and does not interact with individual nutrients during the cultivation cycle.
However, the uptake of particular nutrients varies constantly with plant growth and fruit load and therefore the nutrient balance, pH and EC in the rootzone is also constantly changing.
A regular analysis of this solution, in combination with daily monitoring of EC and pH, allows you to exercise complete control over crop nutrition and plant development. This will not only help avoid serious mistakes (plant and fruit quality) but will also enable you to grow with more precision, optimizing the input of water and fertilizer and minimizing unnecessary flushing of drain water to the environment.
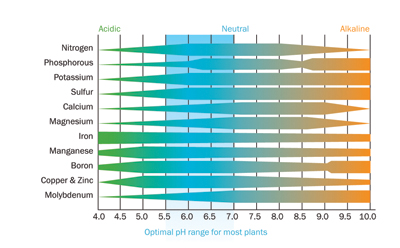 |
| Figure 1: Effect of pH level on the availability of different nutrients.
|
NUTRIENT ANALYSIS IN THE ROOTZONE
■ To ensure regular growth and fruit quality, it is important to maintain the correct balance between individual elements, particularly potassium to calcium (K+:Ca2+) and potassium to nitrogen (K+:NO3–). The rootzone is a dynamic environment that is continually modified by the development of the crop.
A regular analysis of the nutrient solution will ensure that correct and timely adjustments to the drip solution can be made in order to achieve the desired slab target levels. It is advisable to do this on a weekly basis.
 |
|
| Picture 1: Blocked irrigation emitter due to precipitation of calcium phosphate. PHOTOs GROEN AGRO CONTROL, NL |
Taking a sample is easy, but it is a task that must be performed
correctly to secure a representative sample. For an analysis, at least
250 ml of solution is required. Sample flasks should be clean and
completely filled with solution to exclude air. For an analysis of the
slab solution, use a syringe to extract samples from 20 different slabs
from within the irrigation or crop zone.
Take an equal number from under and between the blocks, taking care not to sample too close to the drain hole.
IMPACT OF pH
■ The pH of a solution is a measure of how acidic or alkaline it is, and pH plays an important role as it influences the availability of nutrients (Figure 1). Generally micronutrients iron (Fe3+), manganese (Mn3+) and zinc (Zn2+) become less available as the pH is increased from 6.5 to 7.5 so it is always advisable to supply these in chelated form. At high pH levels, phosphate (H2PO4–) will also precipitate as insoluble calcium phosphate (Figure 2), making phosphate unavailable to the crop as well as blocking the irrigation drippers (Picture 1).
A balanced uptake of each of the nutrient elements is only possible if the pH in the rootzone is within the range of 5.5 to 6.5 (Figure 1). The target pH for the drip solution on stone wool substrates should be 5.5 to 5.8. Never reduce the pH of the drip solution below 5.0; at these levels there is a danger that the acidic solution will break down the stonewool fibres, causing the slabs to collapse.
During cultivation, the pH in the rootzone will fluctuate (Figure 3).
For example, with a greater uptake of negatively charged ions (anions)
such as nitrogen, in response to strong vegetative growth, the pH will
increase (become more alkaline). With a greater uptake of positively
charged ions (cations) such as potassium in response to a high fruit
load, the pH will decrease (become more acidic). To regulate pH, it is
important to maintain the correct balance between ammonium-N and
nitrate-N (NH4+ -N and NO3–-N).
However, the managed changes of pH in the substrate should not be too
large. For this reason, pH should be measured on a daily basis. By doing
so, trends can be followed by plotting results on a graph and you can
react in time by making informed adjustments to the drip pH, or by
adding or removing ammonium nitrate from subsequent nutrient mixes.
Taking a sample is easy, but it is a task that must be performed
correctly to secure a representative sample to avoid making the wrong
conclusions. Simply extract a small quantity of solution from 20 slabs
using a syringe. Don’t mix the samples but measure the pH of each slab
individually below and between the blocks. Please note: drain samples should not be used to determine pH.
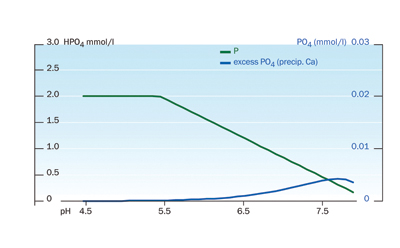 |
| Figure 2: Effect of pH on P availability and precipitation of calcium phosphate.
|
Tips for accurate pH measuring:
- Calibrate the pH meter regularly using standard solutions.
- Check the battery status; low batteries in portable pH meters are often a cause of error.
- Always replace the probe into de-ionized water when not in use.
- Store portable meters in a cool, dry place; do not leave them in the greenhouse.
The following are some tips and actions to take if the pH of the rootzone environment needs adjusting.
The main reasons for a high pH in the rootzone:
- High pH of irrigation water (>5.5).
- Strong vegetative plant growth.
Actions to correct a high pH in the rootzone:
- Decrease the pH of feed solution to 5.2 – 5.3.
- Increase or add ammonium nitrate to the drip solution, i.e., 0.7-1.75 mMol l-1 (10-24 ppm). However for beef tomatoes, use a maximum 1.0 mMol l-1 (14 ppm) ammonium nitrate in the drip solution.
- When you add ammonium nitrate always increase the amount of iron (+20 per cent) as a precautionary measure. If the pH moves >6.0, iron deficiencies can appear rapidly. It would be good practice to use a chelated form of iron with better pH stability – EDTA (pH 3.0-6.0) is most commonly used. However, at higher pH levels, other chelates such as Fe-DTPA or Fe-EDDHA should be considered.
- Increase the length of irrigation cycles and lower their frequency.
- Steer the crop more generatively, i.e., increase the fruit load.
The main reason for a low pH in the rootzone:
- Low pH of the irrigation water <5.3.
- Incorrect composition of nutrient solution, i.e., too much ammonium nitrate or ammonium phosphate.
- Generative plant growth.
Actions to correct a low pH in the rootzone:
- Increase the pH of the nutrient solution to maximum 6.0.
- Decrease the amount of ammonium nitrate in the irrigation water, i.e., <0.7 mMol l-1 (<10 ppm).
- Decrease the length of irrigation cycles and increase the frequency.
- Steer the crop in a vegetative direction, i.e., decrease the fruit load.
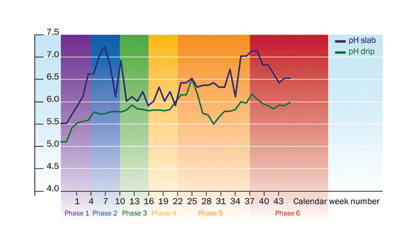 |
| Figure 3: Reaction of drip and slab pH during the cultivation cycle of a tomato crop in Netherlands.
|
IMPACT OF EC
■ EC is a measure of the total salts concentration in the nutrient solution. EC is therefore a mixture of essential (e.g., nitrogen, iron) and non-essential (e.g., sodium, chloride) elements required for plant growth. EC is usually expressed in milliSiemens per linear centimetre (mS/cm) or microSiemens per linear cm (µS/cm) where 1 mS = 1,000 µS. The higher the total salts concentration, the higher the EC. As non-essential elements are not taken up by the plant, they will accumulate in the rootzone over time causing EC to increase. It is therefore important to undertake a regular nutrient analysis to determine the composition of solution.
On a daily basis, EC can be used to influence plant development in line with incumbent weather conditions. For example, when light levels are low, it is possible to work with higher EC in the rootzone to steer the plant in a generative direction. When light levels are high (>1,500J/cm2), a lower EC can be used to steer crop development in a more vegetative direction. EC can also affect yield and fruit quality; in general, a high EC lowers production but increases quality. Maintaining the right balance maximizes economic return to the company.
PLOTTING EC TRENDS TO ADJUST IRRIGATION STRATEGIES
■ The managed changes of EC in the substrate are not likely be too extensive. For this reason, EC should to be measured on a daily basis. By doing so, trends can be followed by plotting the results on a graph and you can react in time by making informed adjustments to the drip EC and irrigation strategy.
A perfect tool for the monitoring the fluctuation in EC and stability of EC during the day in relation to WC and light levels is the WCM-continuous connected to the climate computer (Figure 4). Using this simple graphic, you can zoom in to show in detail how EC reacts over a two- to three-day period, or zoom out to show the trend of EC development over a four- to seven-day period. Alternatively, the hand operated WCM-control can supply these details, both averages of WC and EC as well as logged recordings of a watering section over time.
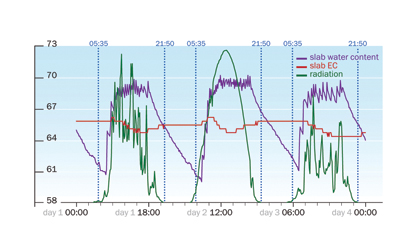 |
| Figure 4: Graphic generated on the climate computer using information from the WCM showing stability of EC in relation the WC and radiation (Wm2).
|
SAMPLE A NUMBER OF SLABS WHEN USING A PORTABLE EC METER
■ When monitoring with a portable EC meter, sampling should take place in a number of slabs so that a meaningful and accurate assessment can be made. Simply extract a small quantity of solution from 20 slabs using a syringe. Don’t mix the samples, but measure the EC of each slab individually. The best time to sample using this method is when the slabs have started to drain, because this is when you want EC to be under control from the irrigation strategy.
In practice, the nutrient solution EC is normally applied in the range 2.5 to 3.5 mS/cm. The target values in the rootzone should be 1.0 to 2.0 mS/cm higher. Greater variation from the target is normally an indication that the irrigation strategy requires adjustment.
Tips for accurate EC measuring:
- Calibrate the EC meter regularly using standard solutions.
- Always take samples from a jar that is clean and preferably rinsed with de-ionized water.
- Always clean the measuring flask after use by rinsing with deionized water.
- Store portable meters in a cool dry place; do not leave them in the greenhouse.
The following are some tips and actions to take if the EC of the rootzone environment needs adjusting.
A high rootzone EC (>5.5 mS/cm)
- Stimulates generative plant development.
- Develops plants with higher DM per cent and less risk of disease.
- Has higher Brix values, promoting better fruit quality.
- In combination with high transpiration by the crop creates a
- higher risk of fruit quality disorders such as blossom end rot (BER).
Actions to correct an EC that is too high:
- Reduce or remove chloride and sulphur fertilizer from the drip solution.
- Reduce drip EC by maximum 0.5 mS/cm and/or use a light reduction EC setting in the irrigation strategy.
- Ensure the irrigation strategy achieves drain on time and supplies correct ml/J throughout the day.
A low rootzone EC (<2. 5 mS/cm)
- Stimulates vegetative plant development.
- Develops plants with low DM per cent and increased risk of soft growth and disease.
- Has lower Brix values, promoting lower fruit quality.
Actions to correct an EC that is too low:
- Add or increase chloride and sulphur fertilizer in the feed mix.
- Increase drip EC by maximum 0.5 mS/cm.
- Do not over supply irrigation water on dark days and be aware that the maximum rest time setting is not too short on dark days.
SUMMARY
■ It is important to maintain a balanced nutrient composition in the slab solution and a controlled and stable pH and EC to achieve the best results and best fruit quality. Monitoring these basic parameters should be obligatory for every grower. This will not only help avoid serious mistakes (plant and fruit quality), but will also enable you to grow with more precision, optimizing input costs of water and fertilizer and minimizing unnecessary flushing.
Andrew Lee works for Grodan BV as Business Support Manager for North America and Export Markets. He is a PhD graduate from the University of London, England.
Print this page